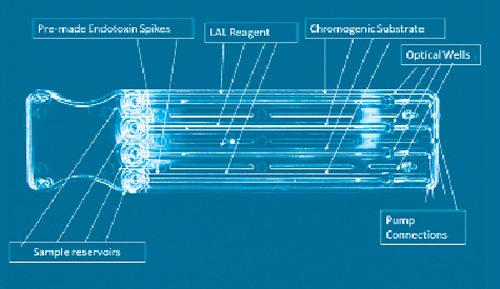
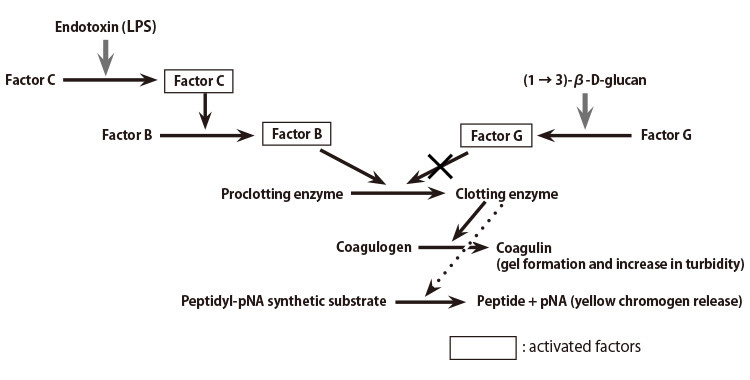
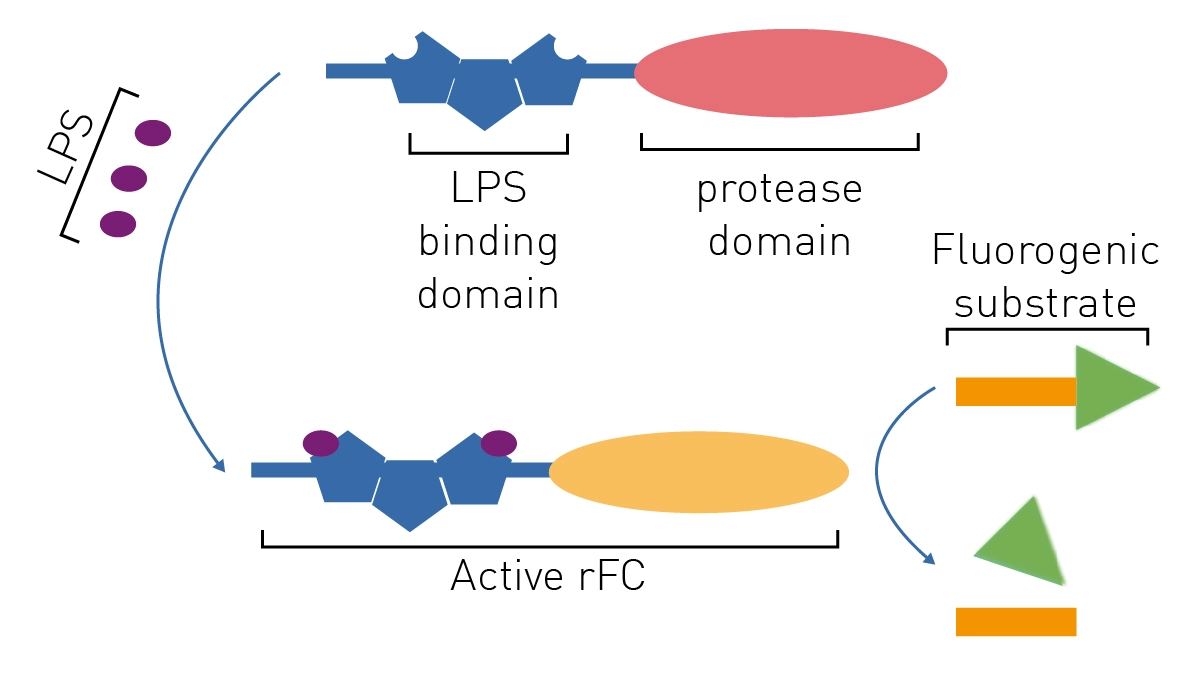
LAL test validation protocol flow-chart. The test was performed three... | Download Scientific Diagram

What Is Limulus Amebocyte Lysate (LAL) and Its Applicability in Endotoxin Quantification of Pharma Products | IntechOpen
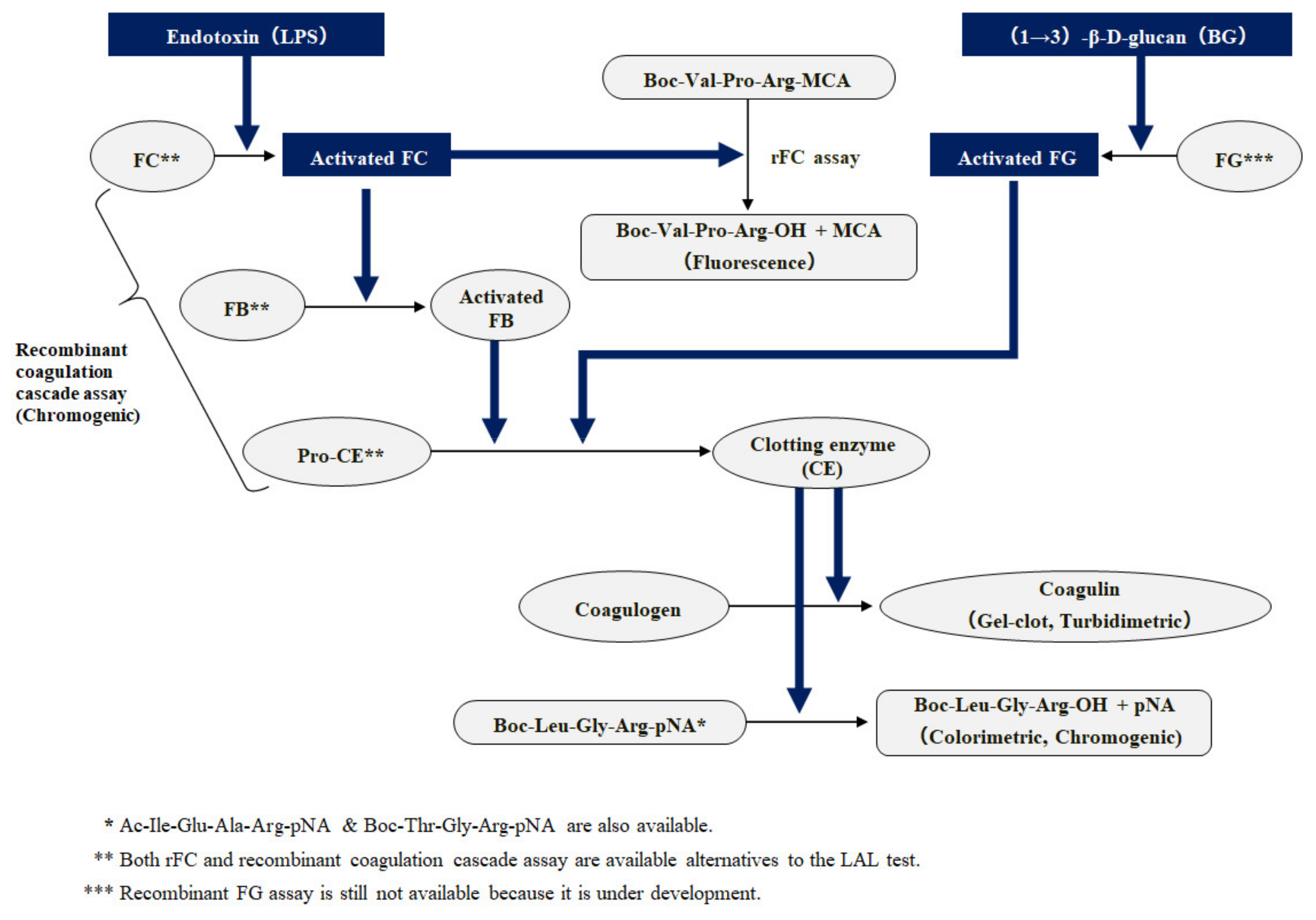
Biomedicines | Free Full-Text | Outstanding Contributions of LAL Technology to Pharmaceutical and Medical Science: Review of Methods, Progress, Challenges, and Future Perspectives in Early Detection and Management of Bacterial Infections and