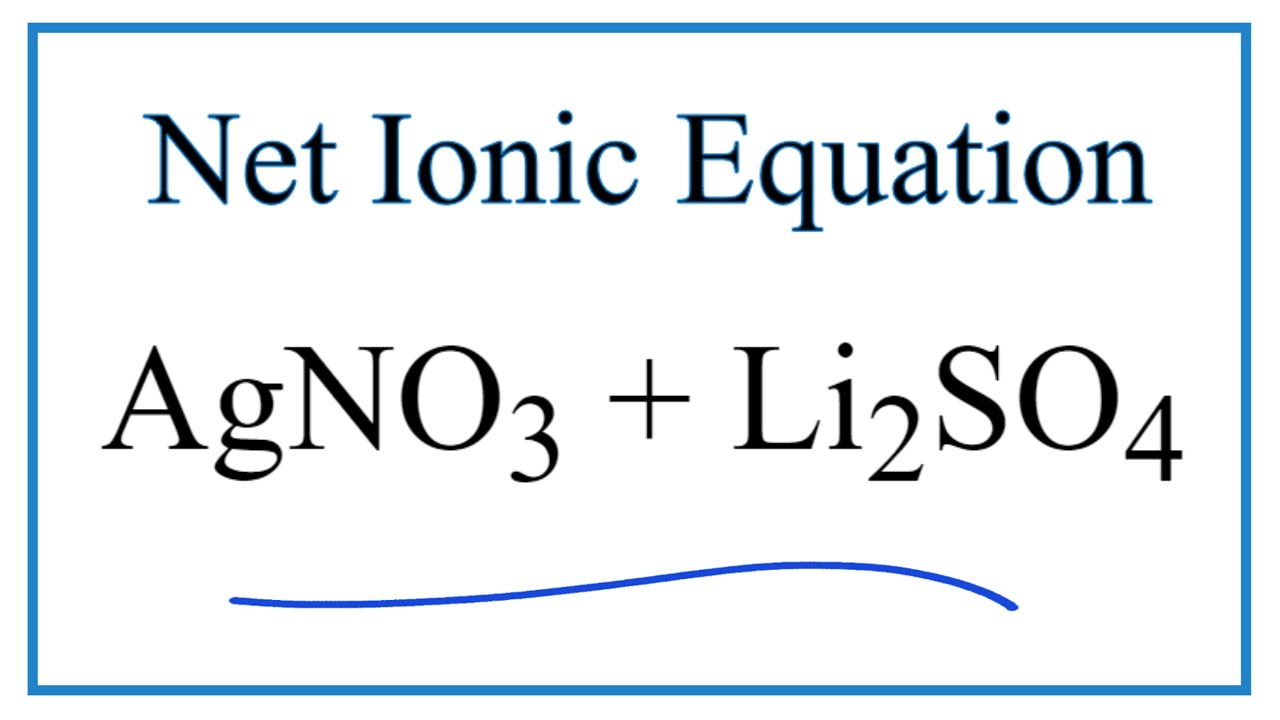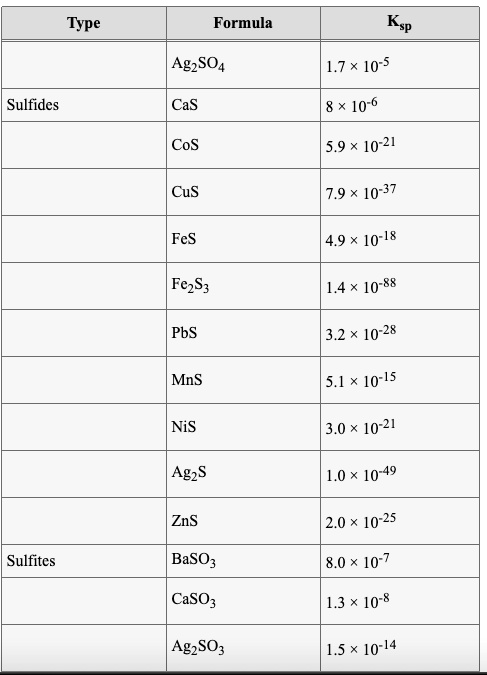
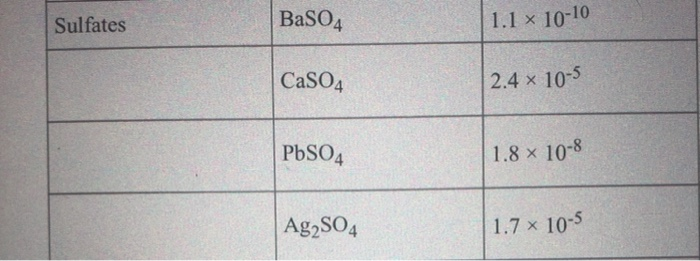
SOLVED: Formula Ag2SO4 L.7 x 10^-5 Sulfides CaS 8 * 10^-6 CoS 5.9 x 10^21 CuS 7.9 x 10^-37 FeS 4.9 x 10^-18 FczS 1.4 x 10^-88 PbS 3.2 x 10^-28 MnS

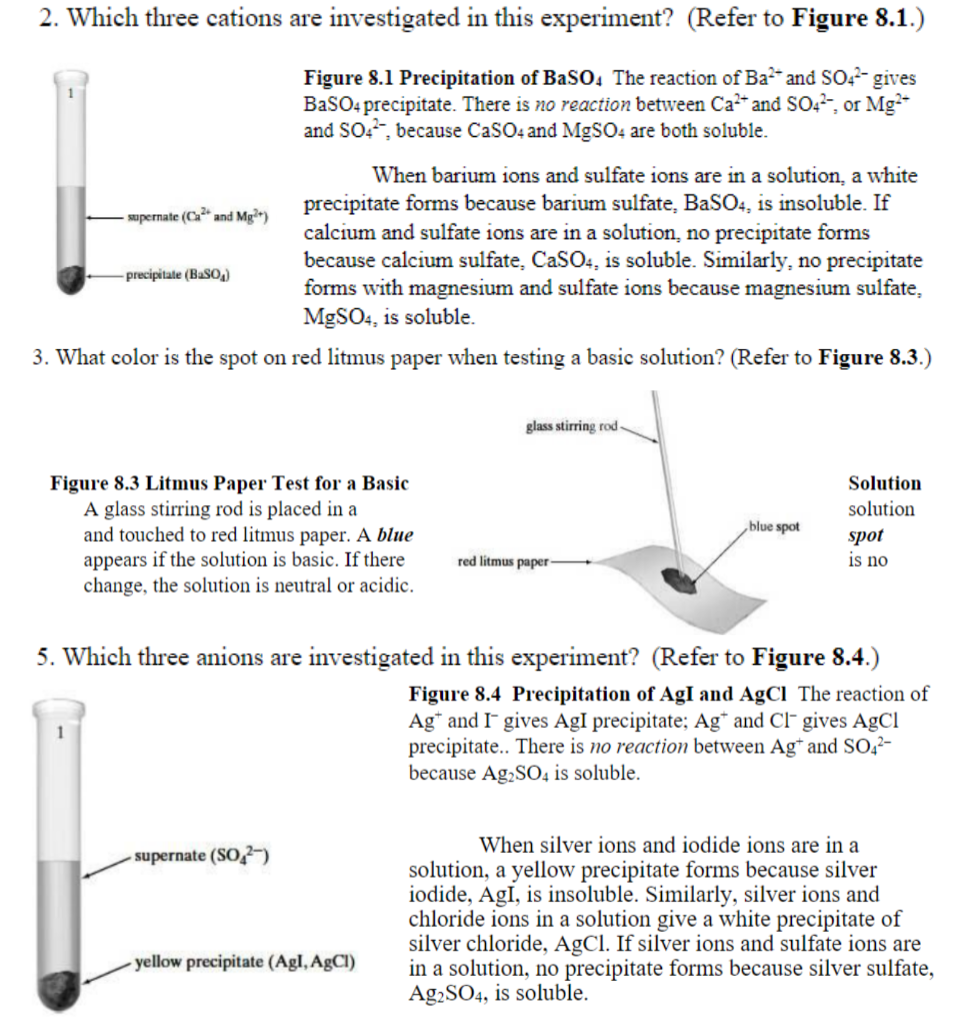
Solubility Allows us to flavor foods -- salt & sugar. Solubility of tooth enamel in acids. Allows use of toxic barium sulfate for intestinal x-rays. - ppt download

Question Video: Identifying an Unknown Solution from the Reaction with a Sodium Sulfite Solution | Nagwa

Ag@Ag2SO4 nanoparticles: simple microwave-assistance synthesis, characterization and its co-photocatalytic degradation of methylene blue | Journal of Materials Science: Materials in Electronics

Copper sulphate and sodium carbonate react with glucose to form a brick-red precipitate. Write the ionic equation for this reaction. | Homework.Study.com
%20at%20room%20temperature%20Iron%20(Fe)%20reacts%20with%20the%20acid%20producing%20hydrogen%20bubbles-%20Fe%20%2B%20H2SO4%20--%20.jpg)
Bildagentur | mauritius images | Iron reacts with sulfuric acid. Iron nail is placed in a test tube with 5M solution of sulfuric acid (H2SO4) at room temperature. Iron (Fe) reacts with
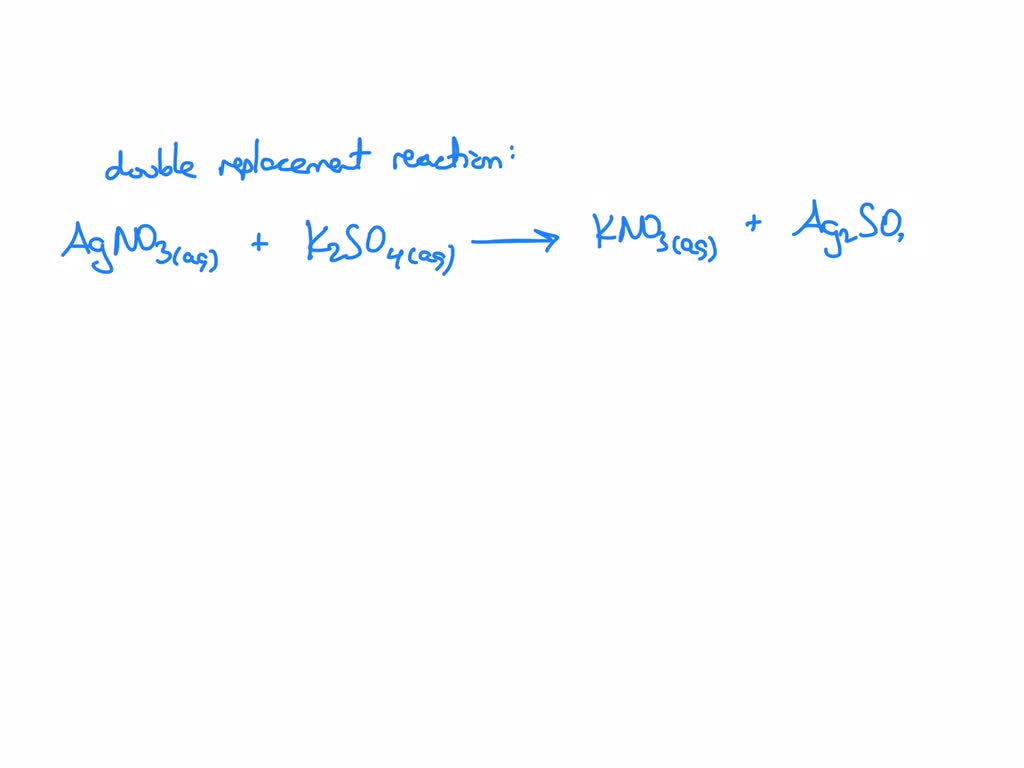
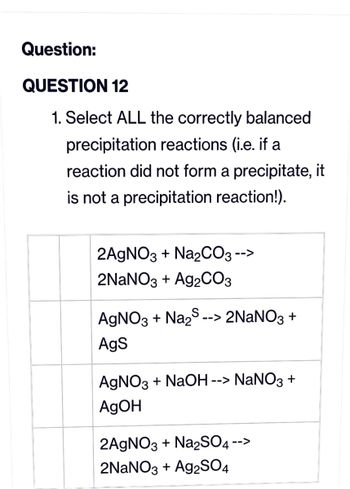
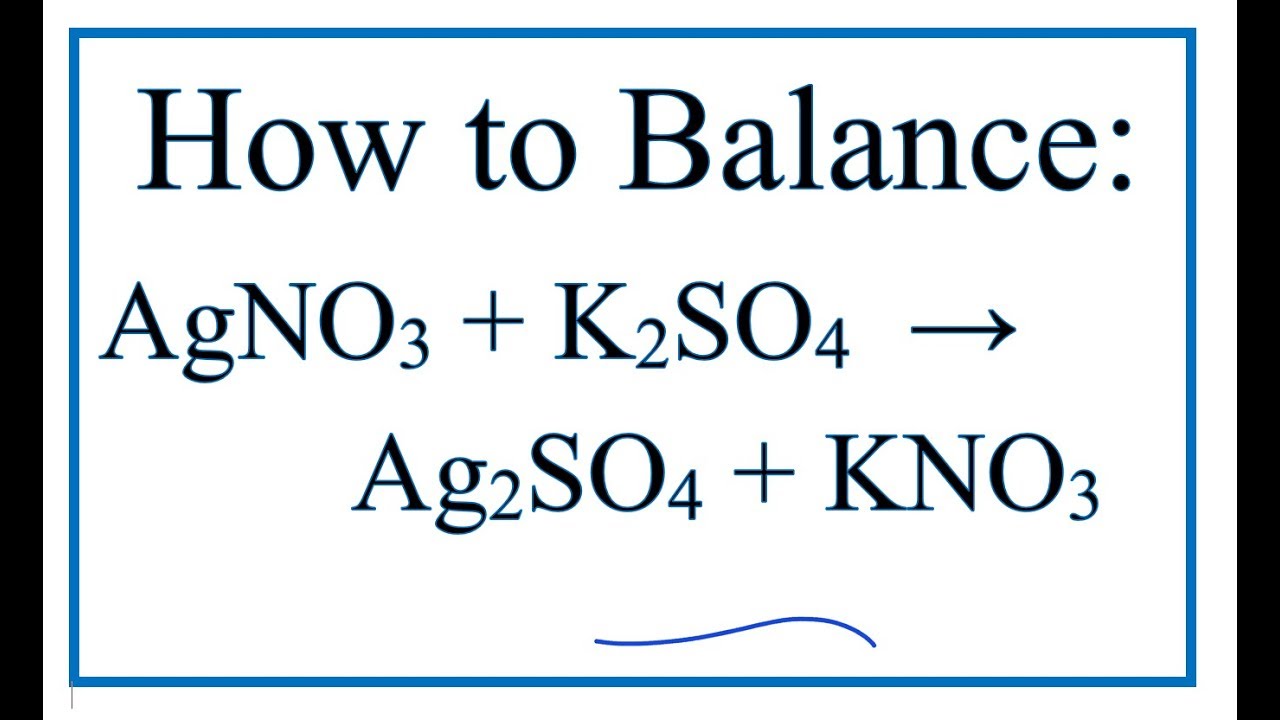
SOLVED: What occurs when aqueous silver nitrate, AgNO3, reacts with aqueous potassium sulfate, K2SO4?' Select one: Ag2SO4 forms as a precipitate. KNO3 forms as a precipitate. K2SO4 forms as a precipitate. No

In the following reaction sequence in aqueous solution, the species X, Y and Z, respectively, are S2032- Ag Ag with time 9 X - Y Clear white black solution precipitate precipitate (A) (

Why does the color of Silver sulphate NPs change from yellow to dark violet without change to red color? | ResearchGate

Transformation from Ag@Ag3PO4 to Ag@Ag2SO4 hybrid at room temperature: preparation and its visible light photocatalytic activity | SpringerLink
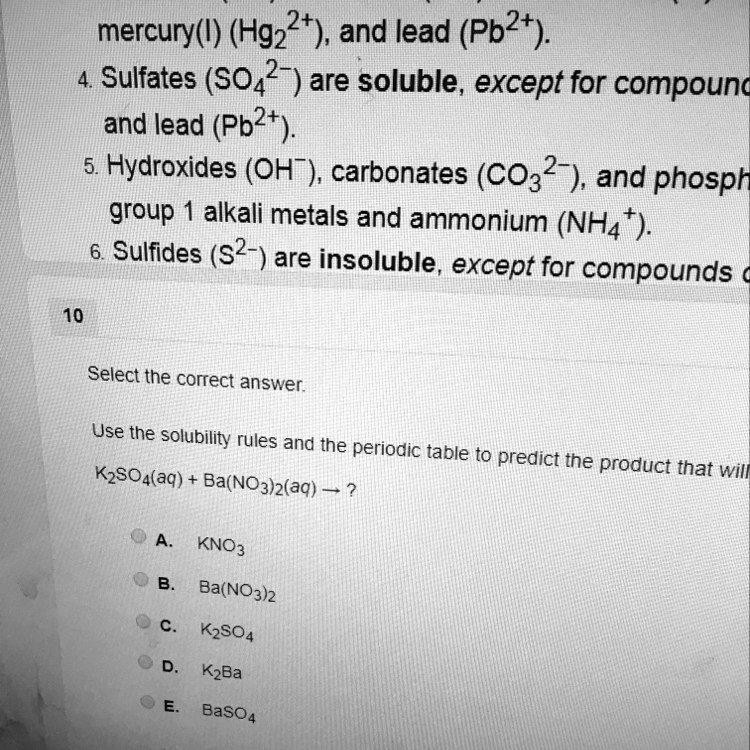

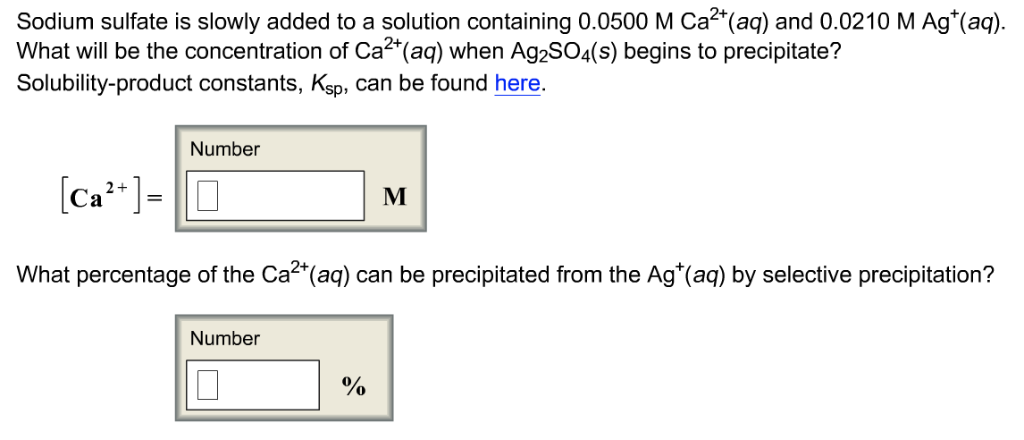


![Ultimate Toolkit for HSC Chemistry Module 8 [Cheatsheet] | Learnable Ultimate Toolkit for HSC Chemistry Module 8 [Cheatsheet] | Learnable](https://www.learnable.education/wp-content/uploads/2020/05/Solubility-Rules.png)